To perform tests SensDx is using Molecular Binding – Electrochemical Impedance Spectroscopy (MB-EIS) technology created over four years of intensive R&D.
Molecular Binding (MB)
SensDx is using patent pending technologies to create stable biofilms capable of binding specific, targeted molecules of tested pathogens or biomarkers. It is a robust approach that allows us to produce biofilms testing for presence of viruses, bacteria, RNA, DNA, oncological markers, etc. The first assay created by the company was Flu SensDx test, with influenza biofilm binding M1 protein. It was CE certified and entered market for 2019.-2020 influenza season.
Electrochemical Impedance Spectroscopy (EIS)
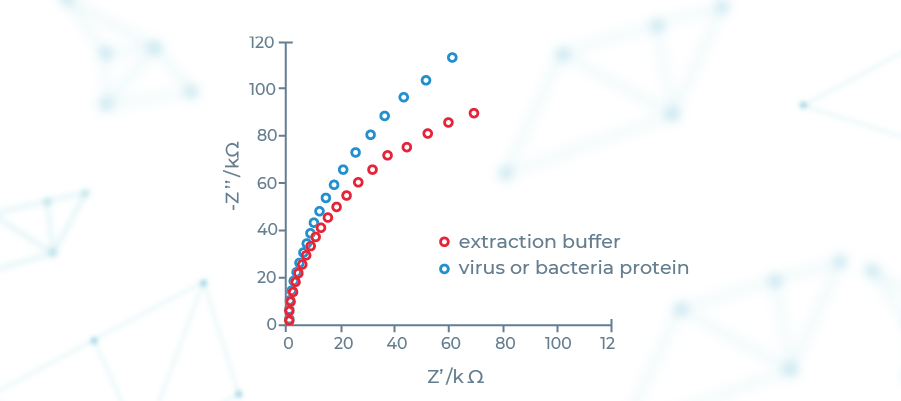
Theoretical foundations of EIS have been laid by Heaviside in the 19th century in the form of Linear Systems Theory (LST). Development of frequency response analyzers in the 1970s have led to it its widespread adoption across number of fields, including life sciences. It is based on introducing a perturbation in the system being studied by using a sine wave current. In SensDx case the system being studied is a patented microsensor containing electrode covered by the biofilm.
We have designed a machine learning supported device that senses the resulting changes of impedance of the microsensor, recognizing specific patterns of target molecules bound to it. This effectively gives us a very precise and versatile diagnostics platform featuring:
Early detection of pathogens or biomarkers
In case of influenza assays SensDx platform is capable of detecting a very small amount of virus. The LOD (Lower Limit of Detection) is as low as in Real-Time PCR (36-38Cq) therefore the test is suitable for the early and late-stage infection diagnosis in opposite to the RIDT tests.
High sensitivity and specificity
Flu SensDx assays have achieved 99% specificity and 94% sensitivity during independent validation studies in comparison to RT-PCR method.
Accurate testing of recovered cases
Whilst RT-PCR is regarded as a gold standard of testing, certain inaccuracies have been identified during the Covid-19 pandemic, where inactive RNA residue would result in false positive diagnosis. SensDx assays are testing for a presence of a live pathogen and do not return false positive results when residual RNA is present in the sample.
Rapid testing at point of care
Whilst tests protocols vary, most of them can be completed within 5-7 minutes right at the point of care, making it a platform of choice for GP surgeries, emergency responders, epidemic control screening at care homes, airports, cruise ships, oil, manufacturing, travel, hospitality and event industries.
Ease of use and accessibility
At current phase of development our platform is targeted for professionals use by medical or paramedical personnel. However, over time, its’ ease of use supported by further platform development will allow for at home testing,










